- Education
-
Research
Current research
Talent
-
Collaboration
Businesses
Government agencies and institutions
Alumni
-
About AU
Organisation
Job at AU
Catalysts made of molybdenum sulfides have been used for the desulphurisation of oil since World War II. Now, Jakob Kibsgaard, however, has revealed promising results to produce hydrogen from water by means of nanoparticles of Mo3S13 in a collaboration between the universities in Aarhus and Stanford. What is so smart about Mo3S13 is that it can be produced cheaply and easily.
2014.01.27 |
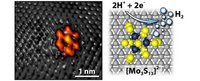
On the left, a scanning tunneling microscope image captures the bright shape of the molybdenum sulfidenanocluster on a graphite surface. The grey spots are carbon atoms. Together the moly sulfide and graphite make the electrode. The diagram on the right shows how two positive hydrogen ions gain electrons through a chemical reaction at the moly sulfide nanocluster to form pure molecular hydrogen. (Image credit Jakob Kibsgaard). 1 nm, nanometer = 10-9 meter
University researchers from two continents have engineered an efficient and environmentally friendly catalyst for the production of molecular hydrogen, a compound used extensively in modern industry to manufacture fertilizer and refine crude oil into gasoline and - possibly - a future substitute for todays fossil fuels.
The experimenters found that their cheap, molybdenum sulfide catalyst had the potential to liberate hydrogen from water on something approaching the efficiency of a system based on prohibitively expensive platinum.