- Education
-
Research
Current research
Talent
-
Collaboration
Businesses
Government agencies and institutions
Alumni
-
About AU
Organisation
Job at AU
By studying a large protein (the C1 protein) with X-rays and electron microscopy, researchers from Aarhus University in Denmark have established a new model for how an important part of the innate immune system is activated. The activation of the C1 protein is a fundamental mechanism in immunology, and therefore the new research results also have medical potential.
2017.01.20 |
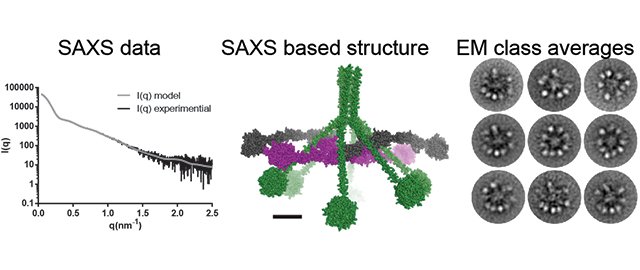
The figure shows the structure of C1 investigated by two different techniques. To the left is shown data recorded by the scientists with X- (black curve) at the PETRA III synchrotron in Hamborg. The grey curve shows how a curve calculated from the model in the middle panel fits to the eksperimental data. To the right is shown so-called class averages of images recorded with electron microskopy. In row two to the right is clearly seen 10 protrusions, which is strictly nono-compatible with the the old model for activation of complement. For this reason the EM-data was pivotal for the results.
An important part of the immune system is the so-called complement system. When the immune system detects a microorganism or other signs of danger, the complement protein C1 is converted into an active enzyme which can cleave other proteins, thus initiating a chain reaction. The end result of this reaction is that, for example, invading pathogenic microorganisms are ingested by our immune cells, and an inflammatory response which leads to the elimination of the microorganisms is triggered. In the past few years, there has been increased focus on the C1 protein, since – in addition to its function in the immune system – it has been shown to be closely involved in the development of the nervous system and neurological disorders.
Textbooks in immunology claim that the C1 protein is activated when a very complicated change in the structure within each C1 molecule takes places when it recognises, for example, a pathogenic organism. The new research results from Aarhus University discard this dogma by showing that activation of the complement system occurs when two C1 proteins are located sufficiently close to each other, which is a much more simple and general mechanism.
The new results were achieved through a close collaboration between four research groups at Aarhus University. First, Postdoctoral Fellow Simon A. Mortensen isolated a special version of the C1 complex with Professor Steffen Thiel from the Department of Biomedicine. Also Professor Jens Christian Jensenius and Laboratory Technician Annette Hansen participated in this work. These samples were analysed with electron microscopy where individual C1 molecules could be identified in collaboration with Associate Professor Bjørn Sander and Associate Professor Monika Golas at the Department of Biomedicine.
"I had carefully optimised my protocol for the preparation of the C1 complex, and it took a long time – at least a year – to make the right sample," says Simon A. Mortensen. "But the first time I saw the C1 molecule clearly with Bjørn and Monika, I was so excited, and it was definitely worth the hard work."
In parallel with this, PhD Student Rasmus K. Jensen used X-rays to examine the structure of Simon’s C1 protein in solution under the guidance of Professor Gregers Rom Andersen from the Department of Molecular Biology and Genetics with the assistance of Professor Jan Skov Pedersen from the iNANO Center. Jan and Simon made the first measurements in Aarhus with X-rays, while Simon, Rasmus and Gregers collected the best data on the PETRA III Synchrotron in Hamburg, and Rasmus then spent months analysing this data.
"The C1 complex was actually too big and complex for our computer programs to calculate its structure," says Rasmus K. Jensen. "So I obtained a special version for this task. Then I went through many cycles of calculations, and each took two weeks, despite the fact that I used powerful computers."
"The C1 complex is the largest and most complicated protein that I have ever worked with for nearly 30 years. Its structure is very unusual so it has been challenging but also really interesting," adds Professor Gregers Rom Andersen.
The results showed that the old model for the activation of the C1 protein had to be discarded because it was simply physically impossible.
"During the past four to five years, we had the feeling that the old model was not correct," says Professor Steffen Thiel. "Also, three years ago, we demonstrated that a corresponding protein with a similar function in another branch of the complement system was activated in the same way. Therefore, with the new results for the C1 protein, we feel confident in suggesting a general model for activation of complement. Thus, we now have a better understanding of how our immune system works," concludes Steffen Thiel.
The research has been published in the renowned journal Proceedings of the National Academy of Sciences USA (PNAS).
Professor Gregers Rom Andersen
Department of Molecular Biology and Genetics
Aarhus University, Denmark
gra@mbg.au.dk - +45 3025 6646
Professor Steffen Thiel
Department of Biomedicine
Aarhus University, Denmark
st@biomed.au.dk - +45 2927 0890